Shared on 29-02-2020
All about Kidney Stons
All about Kidney Stons
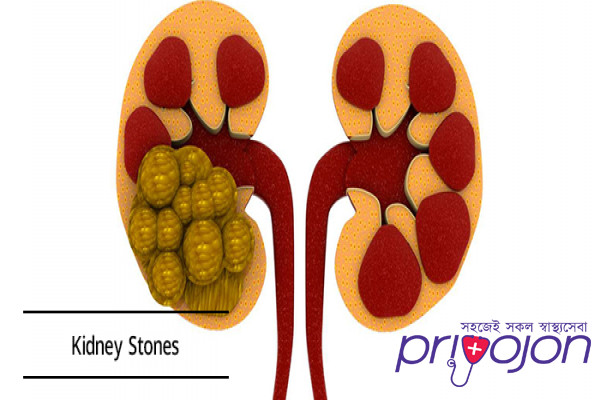
Kidney stone disease, also known as urolithiasis, is when a solid piece of material (kidney stone) develops in the urinary tract.[2] Kidney stones typically form in the kidney and leave the body in the urine stream.[2] A small stone may pass without causing symptoms.[2] If a stone grows to more than 5 millimeters (0.2 in), it can cause blockage of the ureter, resulting in severe pain in the lower back or abdomen.[2][7] A stone may also result in blood in the urine, vomiting, or painful urination.[2] About half of people who have had a kidney stone will have another within ten years.[8]
Most stones form due to a combination of genetics and environmental factors.[2] Risk factors include high urine calcium levels; obesity; certain foods; some medications; calcium supplements; hyperparathyroidism; gout and not drinking enough fluids.[2][8] Stones form in the kidney when minerals in urine are at high concentration.[2] The diagnosis is usually based on symptoms, urine testing, and medical imaging.[2] Blood tests may also be useful.[2] Stones are typically classified by their location: nephrolithiasis (in the kidney), ureterolithiasis (in the ureter), cystolithiasis (in the bladder), or by what they are made of (calcium oxalate, uric acid, struvite, cystine).[2]
In those who have had stones, prevention is by drinking fluids such that more than two liters of urine are produced per day.[4] If this is not effective enough, thiazide diuretic, citrate, or allopurinol may be taken.[4] It is recommended that soft drinks containing phosphoric acid (typically colas) be avoided.[4] When a stone causes no symptoms, no treatment is needed.[2] Otherwise pain control is usually the first measure, using medications such as nonsteroidal anti-inflammatory drugs or opioids.[7][9] Larger stones may be helped to pass with the medication tamsulosin[10] or may require procedures such as extracorporeal shock wave lithotripsy, ureteroscopy, or percutaneous nephrolithotomy.[2]
Between 1% and 15% of people globally are affected by kidney stones at some point in their lives.[8] In 2015, 22.1 million cases occurred,[5] resulting in about 16,100 deaths.[6] They have become more common in the Western world since the 1970s.[8] Generally, more men are affected than women.[2] Kidney stones have affected humans throughout history with descriptions of surgery to remove them dating from as early as 600 BC.[1]
Signs and symptoms
The hallmark of a stone that obstructs the ureter or renal pelvis is excruciating, intermittent pain that radiates from the flank to the groin or to the inner thigh.[11] This pain, known as renal colic, is often described as one of the strongest pain sensations known.[12] Renal colic caused by kidney stones is commonly accompanied by urinary urgency, restlessness, hematuria, sweating, nausea, and vomiting. It typically comes in waves lasting 20 to 60 minutes caused by peristaltic contractions of the ureter as it attempts to expel the stone.[11]
The embryological link between the urinary tract, the genital system, and the gastrointestinal tract is the basis of the radiation of pain to the gonads, as well as the nausea and vomiting that are also common in urolithiasis.[13] Postrenal azotemia and hydronephrosis can be observed following the obstruction of urine flow through one or both ureters.[14]
Pain in the lower-left quadrant can sometimes be confused with diverticulitis because the sigmoid colon overlaps the ureter, and the exact location of the pain may be difficult to isolate due to the proximity of these two structures.
Risk factors
Dehydration from low fluid intake is a major factor in stone formation.[11][15] Individuals living in warm climates are at higher risk due to increased fluid loss.[16] Obesity, immobility, and sedentary lifestyles are other leading risk factors.[16]
High dietary intake of animal protein,[11] sodium, sugars including honey, refined sugars, fructose and high fructose corn syrup,[17] and excessive consumption of tea or fruit juices may increase the risk of kidney stone formation due to increased uric acid excretion and elevated urinary oxalate levels.[16][15]
Kidney stones can result from an underlying metabolic condition, such as distal renal tubular acidosis,[18] Dent's disease,[19] hyperparathyroidism,[20] primary hyperoxaluria,[21] or medullary sponge kidney. 3–20% of people who form kidney stones have medullary sponge kidney.[22][23]
Kidney stones are more common in people with Crohn's disease;[24] Crohn's disease is associated with hyperoxaluria and malabsorption of magnesium.[25]
A person with recurrent kidney stones may be screened for such disorders. This is typically done with a 24-hour urine collection. The urine is analyzed for features that promote stone formation.[14]
Calcium oxalate
Calcium is one component of the most common type of human kidney stones, calcium oxalate. Some studies[which?] suggest that people who take calcium or vitamin D as a dietary supplement have a higher risk of developing kidney stones. In the United States, kidney stone formation was used as an indicator of excess calcium intake by the Reference Daily Intake committee for calcium in adults.[26]
In the early 1990s, a study conducted for the Women's Health Initiative in the US found that postmenopausal women who consumed 1000 mg of supplemental calcium and 400 international units of vitamin D per day for seven years had a 17% higher risk of developing kidney stones than subjects taking a placebo.[27] The Nurses' Health Study also showed an association between supplemental calcium intake and kidney stone formation.[28]
Unlike supplemental calcium, high intakes of dietary calcium do not appear to cause kidney stones and may actually protect against their development.[28][27] This is perhaps related to the role of calcium in binding ingested oxalate in the gastrointestinal tract. As the amount of calcium intake decreases, the amount of oxalate available for absorption into the bloodstream increases; this oxalate is then excreted in greater amounts into the urine by the kidneys. In the urine, oxalate is a very strong promoter of calcium oxalate precipitation—about 15 times stronger than calcium.
A 2004 study found that diets low in calcium are associated with a higher overall risk for kidney stone formation.[29] For most individuals, other risk factors for kidney stones, such as high intakes of dietary oxalates and low fluid intake, play a greater role than calcium intake.[30]
Other electrolytes
Calcium is not the only electrolyte that influences the formation of kidney stones. For example, by increasing urinary calcium excretion, high dietary sodium may increase the risk of stone formation.[28]
Drinking fluoridated tap water may increase the risk of kidney stone formation by a similar mechanism, though further epidemiologic studies are warranted to determine whether fluoride in drinking water is associated with an increased incidence of kidney stones.[31] High dietary intake of potassium appears to reduce the risk of stone formation because potassium promotes the urinary excretion of citrate, an inhibitor of calcium crystal formation.[32]
Kidney stones are more likely to develop, and to grow larger, if a person has low dietary magnesium. Magnesium inhibits stone formation.[33]
Animal protein
Diets in Western nations typically contain a large proportion of animal protein. Eating animal protein creates an acid load that increases urinary excretion of calcium and uric acid and reduced citrate. Urinary excretion of excess sulfurous amino acids (e.g., cysteine and methionine), uric acid, and other acidic metabolites from animal protein acidifies the urine, which promotes the formation of kidney stones.[34] Low urinary-citrate excretion is also commonly found in those with a high dietary intake of animal protein, whereas vegetarians tend to have higher levels of citrate excretion.[28] Low urinary citrate, too, promotes stone formation.[34]
Vitamins
The evidence linking vitamin C supplements with an increased rate of kidney stones is inconclusive.[35][36] The excess dietary intake of vitamin C might increase the risk of calcium-oxalate stone formation.[37] The link between vitamin D intake and kidney stones is also tenuous. Excessive vitamin D supplementation may increase the risk of stone formation by increasing the intestinal absorption of calcium; correction of a deficiency does not.[28]
Other
There are no conclusive data demonstrating a cause-and-effect relationship between alcoholic beverage consumption and kidney stones. However, some people have theorized that certain behaviors associated with frequent and binge drinking can lead to dehydration, which can, in turn, lead to the development of kidney stones.[38]
The American Urological Association has projected that global warming will lead to an increased incidence of kidney stones in the United States by expanding the "kidney stone belt" of the southern United States.[39]
In one study, people with lymphoproliferative/myeloproliferative disorders who were treated with chemotherapy developed symptomatic kidney stones 1.8% of the time.[40]
Prevention
Preventative measures depend on the type of stones. In those with calcium stones, drinking lots of fluids, thiazide diuretics and citrate are effective as is allopurinol in those with high uric acid levels in the blood or urine.[75][76]
Dietary measures
Specific therapy should be tailored to the type of stones involved. Diet can have an effect on the development of kidney stones. Preventive strategies include some combination of dietary modifications and medications with the goal of reducing the excretory load of calculogenic compounds on the kidneys.[29][77][78] Dietary recommendations to minimize the formation of kidney stones include:
Increasing total fluid intake to more than two liters per day of urine output.[79]
Limiting cola, including sugar-sweetened soft drinks,[75][79][80] to less than one liter per week.[81]
Limiting animal protein intake to no more than two meals daily (an association between animal protein and recurrence of kidney stones has been shown in men[82]).
Maintenance of dilute urine by means of vigorous fluid therapy is beneficial in all forms of kidney stones, so increasing urine volume is a key principle for the prevention of kidney stones. Fluid intake should be sufficient to maintain a urine output of at least 2 litres (68 US fl oz) per day.[76] A high fluid intake has been associated with a 40% reduction in recurrence risk.[52] The quality of the evidence for this, however, is not very good.[76]
Calcium binds with available oxalate in the gastrointestinal tract, thereby preventing its absorption into the bloodstream, and reducing oxalate absorption decreases kidney stone risk in susceptible people.[83] Because of this, some doctors recommend chewing calcium tablets during meals containing oxalate foods.[84] Calcium citrate supplements can be taken with meals if dietary calcium cannot be increased by other means. The preferred calcium supplement for people at risk of stone formation is calcium citrate because it helps to increase urinary citrate excretion.[78]
Aside from vigorous oral hydration and eating more dietary calcium, other prevention strategies include avoidance of large doses of supplemental vitamin C and restriction of oxalate-rich foods such as leaf vegetables, rhubarb, soy products and chocolate.[85] However, no randomized, controlled trial of oxalate restriction has been performed to test the hypothesis that oxalate restriction reduces stone formation.[84] Some evidence indicates magnesium intake decreases the risk of symptomatic kidney stones.[85]
Urine alkalinization
The mainstay for medical management of uric acid stones is alkalinization (increasing the pH) of the urine. Uric acid stones are among the few types amenable to dissolution therapy, referred to as chemolysis. Chemolysis is usually achieved through the use of oral medications, although in some cases, intravenous agents or even instillation of certain irrigating agents directly onto the stone can be performed, using antegrade nephrostomy or retrograde ureteral catheters.[43] Acetazolamide is a medication that alkalinizes the urine. In addition to acetazolamide or as an alternative, certain dietary supplements are available that produce a similar alkalinization of the urine. These include sodium bicarbonate, potassium citrate, magnesium citrate, and Bicitra (a combination of citric acid monohydrate and sodium citrate dihydrate).[86] Aside from alkalinization of the urine, these supplements have the added advantage of increasing the urinary citrate level, which helps to reduce the aggregation of calcium oxalate stones.[43]
Increasing the urine pH to around 6.5 provides optimal conditions for dissolution of uric acid stones. Increasing the urine pH to a value higher than 7.0 increases the risk of calcium phosphate stone formation. Testing the urine periodically with nitrazine paper can help to ensure the urine pH remains in this optimal range. Using this approach, stone dissolution rate can be expected to be around 10 mm (0.4 in) of stone radius per month.[43]
Diuretics
One of the recognized medical therapies for prevention of stones is the thiazide and thiazide-like diuretics, such as chlorthalidone or indapamide. These drugs inhibit the formation of calcium-containing stones by reducing urinary calcium excretion.[11] Sodium restriction is necessary for clinical effect of thiazides, as sodium excess promotes calcium excretion. Thiazides work best for renal leak hypercalciuria (high urine calcium levels), a condition in which high urinary calcium levels are caused by a primary kidney defect. Thiazides are useful for treating absorptive hypercalciuria, a condition in which high urinary calcium is a result of excess absorption from the gastrointestinal tract.[45]
Allopurinol
For people with hyperuricosuria and calcium stones, allopurinol is one of the few treatments that have been shown to reduce kidney stone recurrences. Allopurinol interferes with the production of uric acid in the liver. The drug is also used in people with gout or hyperuricemia (high serum uric acid levels).[87] Dosage is adjusted to maintain a reduced urinary excretion of uric acid. Serum uric acid level at or below 6 mg/100 ml) is often a therapeutic goal. Hyperuricemia is not necessary for the formation of uric acid stones; hyperuricosuria can occur in the presence of normal or even low serum uric acid. Some practitioners advocate adding allopurinol only in people in whom hyperuricosuria and hyperuricemia persist, despite the use of a urine-alkalinizing agent such as sodium bicarbonate or potassium citrate.[43]
Treatment
Stone size influences the rate of spontaneous stone passage. For example, up to 98% of small stones (less than 5 mm (0.2 in) in diameter) may pass spontaneously through urination within four weeks of the onset of symptoms,[7] but for larger stones (5 to 10 mm (0.2 to 0.4 in) in diameter), the rate of spontaneous passage decreases to less than 53%.[73] Initial stone location also influences the likelihood of spontaneous stone passage. Rates increase from 48% for stones located in the proximal ureter to 79% for stones located at the vesicoureteric junction, regardless of stone size.[73] Assuming no high-grade obstruction or associated infection is found in the urinary tract, and symptoms are relatively mild, various nonsurgical measures can be used to encourage the passage of a stone.[43] Repeat stone formers benefit from more intense management, including proper fluid intake and use of certain medications, as well as careful monitoring.[88]
Pain management
Management of pain often requires intravenous administration of NSAIDs or opioids.[11] NSAIDs appear somewhat better than opioids or paracetamol in those with normal kidney function.[89] Medications by mouth are often effective for less severe discomfort.[56] The use of antispasmodics does not have further benefit.[9]
Medical expulsive therapy
The use of medications to speed the spontaneous passage of stones in the ureter is referred to as medical expulsive therapy.[90][91] Several agents, including alpha adrenergic blockers (such as tamsulosin) and calcium channel blockers (such as nifedipine), may be effective.[90] Alpha-blockers likely result in more people passing their stones, and they may pass their stones in a shorter time.[91] Alpha-blockers appear to be more effective for larger stones (over 5 mm in size) than smaller stones.[91] A combination of tamsulosin and a corticosteroid may be better than tamsulosin alone.[90] These treatments also appear to be a useful in addition to lithotripsy.[7]
Lithotripsy
Extracorporeal shock wave lithotripsy (ESWL) is a noninvasive technique for the removal of kidney stones. Most ESWL is carried out when the stone is present near the renal pelvis. ESWL involves the use of a lithotriptor machine to deliver externally applied, focused, high-intensity pulses of ultrasonic energy to cause fragmentation of a stone over a period of around 30–60 minutes. Following its introduction in the United States in February 1984, ESWL was rapidly and widely accepted as a treatment alternative for renal and ureteral stones.[92] It is currently used in the treatment of uncomplicated stones located in the kidney and upper ureter, provided the aggregate stone burden (stone size and number) is less than 20 mm (0.8 in) and the anatomy of the involved kidney is normal.[93][94]
For a stone greater than 10 mm (0.4 in), ESWL may not help break the stone in one treatment; instead, two or three treatments may be needed. Some 80 to 85% of simple renal calculi can be effectively treated with ESWL.[7] A number of factors can influence its efficacy, including chemical composition of the stone, presence of anomalous renal anatomy and the specific location of the stone within the kidney, presence of hydronephrosis, body mass index, and distance of the stone from the surface of the skin.[92] Common adverse effects of ESWL include acute trauma, such as bruising at the site of shock administration, and damage to blood vessels of the kidney.[95][96] In fact, the vast majority of people who are treated with a typical dose of shock waves using currently accepted treatment settings are likely to experience some degree of acute kidney injury.[92]
ESWL-induced acute kidney injury is dose-dependent (increases with the total number of shock waves administered and with the power setting of the lithotriptor) and can be severe,[92] including internal bleeding and subcapsular hematomas. On rare occasions, such cases may require blood transfusion and even lead to acute kidney failure. Hematoma rates may be related to the type of lithotriptor used; hematoma rates of less than 1% and up to 13% have been reported for different lithotriptor machines.[96] Recent studies show reduced acute tissue injury when the treatment protocol includes a brief pause following the initiation of treatment, and both improved stone breakage and a reduction in injury when ESWL is carried out at slow shock wave rate.[92]
In addition to the aforementioned potential for acute kidney injury, animal studies suggest these acute injuries may progress to scar formation, resulting in loss of functional renal volume.[95][96] Recent prospective studies also indicate elderly people are at increased risk of developing new-onset hypertension following ESWL. In addition, a retrospective case-control study published by researchers from the Mayo Clinic in 2006 has found an increased risk of developing diabetes mellitus and hypertension in people who had undergone ESWL, compared with age and gender-matched people who had undergone nonsurgical treatment. Whether or not acute trauma progresses to long-term effects probably depends on multiple factors that include the shock wave dose (i.e., the number of shock waves delivered, rate of delivery, power setting, acoustic characteristics of the particular lithotriptor, and frequency of retreatment), as well as certain intrinsic predisposing pathophysiologic risk factors.[92]
To address these concerns, the American Urological Association established the Shock Wave Lithotripsy Task Force to provide an expert opinion on the safety and risk-benefit ratio of ESWL. The task force published a white paper outlining their conclusions in 2009. They concluded the risk-benefit ratio remains favorable for many people.[92] The advantages of ESWL include its noninvasive nature, the fact that it is technically easy to treat most upper urinary tract calculi, and that, at least acutely, it is a well-tolerated, low-morbidity treatment for the vast majority of people. However, they recommended slowing the shock wave firing rate from 120 pulses per minute to 60 pulses per minute to reduce the risk of renal injury and increase the degree of stone fragmentation.[92]
Surgery
Most stones under 5 mm (0.2 in) pass spontaneously.[29][7] Prompt surgery may, nonetheless, be required in persons with only one working kidney, bilateral obstructing stones, a urinary tract infection and thus, it is presumed, an infected kidney, or intractable pain.[97] Beginning in the mid-1980s, less invasive treatments such as extracorporeal shock wave lithotripsy, ureteroscopy, and percutaneous nephrolithotomy began to replace open surgery as the modalities of choice for the surgical management of urolithiasis.[7] More recently, flexible ureteroscopy has been adapted to facilitate retrograde nephrostomy creation for percutaneous nephrolithotomy. This approach is still under investigation, though early results are favorable.[98] Percutaneous nephrolithotomy or, rarely, anatrophic nephrolithotomy, is the treatment of choice for large or complicated stones (such as calyceal staghorn calculi) or stones that cannot be extracted using less invasive procedures.[47][7]
Ureteroscopic surgery
Ureteroscopy has become increasingly popular as flexible and rigid fiberoptic ureteroscopes have become smaller. One ureteroscopic technique involves the placement of a ureteral stent (a small tube extending from the bladder, up the ureter and into the kidney) to provide immediate relief of an obstructed kidney. Stent placement can be useful for saving a kidney at risk for postrenal acute kidney failure due to the increased hydrostatic pressure, swelling and infection (pyelonephritis and pyonephrosis) caused by an obstructing stone. Ureteral stents vary in length from 24 to 30 cm (9.4 to 11.8 in) and most have a shape commonly referred to as a "double-J" or "double pigtail", because of the curl at both ends. They are designed to allow urine to flow past an obstruction in the ureter. They may be retained in the ureter for days to weeks as infections resolve and as stones are dissolved or fragmented by ESWL or by some other treatment. The stents dilate the ureters, which can facilitate instrumentation, and they also provide a clear landmark to aid in the visualization of the ureters and any associated stones on radiographic examinations. The presence of indwelling ureteral stents may cause minimal to moderate discomfort, frequency or urgency incontinence, and infection, which in general resolves on removal. Most ureteral stents can be removed cystoscopically during an office visit under topical anesthesia after resolution of urolithiasis.[99]
More definitive ureteroscopic techniques for stone extraction (rather than simply bypassing the obstruction) include basket extraction and ultrasound ureterolithotripsy. Laser lithotripsy is another technique, which involves the use of a holmium:yttrium aluminium garnet (Ho:YAG) laser to fragment stones in the bladder, ureters, and kidneys.[100]
Ureteroscopic techniques are generally more effective than ESWL for treating stones located in the lower ureter, with success rates of 93–100% using Ho:YAG laser lithotripsy.[73] Although ESWL has been traditionally preferred by many practitioners for treating stones located in the upper ureter, more recent experience suggests ureteroscopic techniques offer distinct advantages in the treatment of upper ureteral stones. Specifically, the overall success rate is higher, fewer repeat interventions and postoperative visits are needed, and treatment costs are lower after ureteroscopic treatment when compared with ESWL. These advantages are especially apparent with stones greater than 10 mm (0.4 in) in diameter. However, because ureteroscopy of the upper ureter is much more challenging than ESWL, many urologists still prefer to use ESWL as a first-line treatment for stones of less than 10 mm, and ureteroscopy for those greater than 10 mm in diameter.[73] Ureteroscopy is the preferred treatment in pregnant and morbidly obese people, as well as those with bleeding disorders.[7]
Kidney stone disease, also known as urolithiasis, is when a solid piece of material (kidney stone) develops in the urinary tract.[2] Kidney stones typically form in the kidney and leave the body in the urine stream.[2] A small stone may pass without causing symptoms.[2] If a stone grows to more than 5 millimeters (0.2 in), it can cause blockage of the ureter, resulting in severe pain in the lower back or abdomen.[2][7] A stone may also result in blood in the urine, vomiting, or painful urination.[2] About half of people who have had a kidney stone will have another within ten years.[8]
Most stones form due to a combination of genetics and environmental factors.[2] Risk factors include high urine calcium levels; obesity; certain foods; some medications; calcium supplements; hyperparathyroidism; gout and not drinking enough fluids.[2][8] Stones form in the kidney when minerals in urine are at high concentration.[2] The diagnosis is usually based on symptoms, urine testing, and medical imaging.[2] Blood tests may also be useful.[2] Stones are typically classified by their location: nephrolithiasis (in the kidney), ureterolithiasis (in the ureter), cystolithiasis (in the bladder), or by what they are made of (calcium oxalate, uric acid, struvite, cystine).[2]
In those who have had stones, prevention is by drinking fluids such that more than two liters of urine are produced per day.[4] If this is not effective enough, thiazide diuretic, citrate, or allopurinol may be taken.[4] It is recommended that soft drinks containing phosphoric acid (typically colas) be avoided.[4] When a stone causes no symptoms, no treatment is needed.[2] Otherwise pain control is usually the first measure, using medications such as nonsteroidal anti-inflammatory drugs or opioids.[7][9] Larger stones may be helped to pass with the medication tamsulosin[10] or may require procedures such as extracorporeal shock wave lithotripsy, ureteroscopy, or percutaneous nephrolithotomy.[2]
Between 1% and 15% of people globally are affected by kidney stones at some point in their lives.[8] In 2015, 22.1 million cases occurred,[5] resulting in about 16,100 deaths.[6] They have become more common in the Western world since the 1970s.[8] Generally, more men are affected than women.[2] Kidney stones have affected humans throughout history with descriptions of surgery to remove them dating from as early as 600 BC.[1]
Signs and symptoms
The hallmark of a stone that obstructs the ureter or renal pelvis is excruciating, intermittent pain that radiates from the flank to the groin or to the inner thigh.[11] This pain, known as renal colic, is often described as one of the strongest pain sensations known.[12] Renal colic caused by kidney stones is commonly accompanied by urinary urgency, restlessness, hematuria, sweating, nausea, and vomiting. It typically comes in waves lasting 20 to 60 minutes caused by peristaltic contractions of the ureter as it attempts to expel the stone.[11]
The embryological link between the urinary tract, the genital system, and the gastrointestinal tract is the basis of the radiation of pain to the gonads, as well as the nausea and vomiting that are also common in urolithiasis.[13] Postrenal azotemia and hydronephrosis can be observed following the obstruction of urine flow through one or both ureters.[14]
Pain in the lower-left quadrant can sometimes be confused with diverticulitis because the sigmoid colon overlaps the ureter, and the exact location of the pain may be difficult to isolate due to the proximity of these two structures.
Risk factors
Dehydration from low fluid intake is a major factor in stone formation.[11][15] Individuals living in warm climates are at higher risk due to increased fluid loss.[16] Obesity, immobility, and sedentary lifestyles are other leading risk factors.[16]
High dietary intake of animal protein,[11] sodium, sugars including honey, refined sugars, fructose and high fructose corn syrup,[17] and excessive consumption of tea or fruit juices may increase the risk of kidney stone formation due to increased uric acid excretion and elevated urinary oxalate levels.[16][15]
Kidney stones can result from an underlying metabolic condition, such as distal renal tubular acidosis,[18] Dent's disease,[19] hyperparathyroidism,[20] primary hyperoxaluria,[21] or medullary sponge kidney. 3–20% of people who form kidney stones have medullary sponge kidney.[22][23]
Kidney stones are more common in people with Crohn's disease;[24] Crohn's disease is associated with hyperoxaluria and malabsorption of magnesium.[25]
A person with recurrent kidney stones may be screened for such disorders. This is typically done with a 24-hour urine collection. The urine is analyzed for features that promote stone formation.[14]
Calcium oxalate
Calcium is one component of the most common type of human kidney stones, calcium oxalate. Some studies[which?] suggest that people who take calcium or vitamin D as a dietary supplement have a higher risk of developing kidney stones. In the United States, kidney stone formation was used as an indicator of excess calcium intake by the Reference Daily Intake committee for calcium in adults.[26]
In the early 1990s, a study conducted for the Women's Health Initiative in the US found that postmenopausal women who consumed 1000 mg of supplemental calcium and 400 international units of vitamin D per day for seven years had a 17% higher risk of developing kidney stones than subjects taking a placebo.[27] The Nurses' Health Study also showed an association between supplemental calcium intake and kidney stone formation.[28]
Unlike supplemental calcium, high intakes of dietary calcium do not appear to cause kidney stones and may actually protect against their development.[28][27] This is perhaps related to the role of calcium in binding ingested oxalate in the gastrointestinal tract. As the amount of calcium intake decreases, the amount of oxalate available for absorption into the bloodstream increases; this oxalate is then excreted in greater amounts into the urine by the kidneys. In the urine, oxalate is a very strong promoter of calcium oxalate precipitation—about 15 times stronger than calcium.
A 2004 study found that diets low in calcium are associated with a higher overall risk for kidney stone formation.[29] For most individuals, other risk factors for kidney stones, such as high intakes of dietary oxalates and low fluid intake, play a greater role than calcium intake.[30]
Other electrolytes
Calcium is not the only electrolyte that influences the formation of kidney stones. For example, by increasing urinary calcium excretion, high dietary sodium may increase the risk of stone formation.[28]
Drinking fluoridated tap water may increase the risk of kidney stone formation by a similar mechanism, though further epidemiologic studies are warranted to determine whether fluoride in drinking water is associated with an increased incidence of kidney stones.[31] High dietary intake of potassium appears to reduce the risk of stone formation because potassium promotes the urinary excretion of citrate, an inhibitor of calcium crystal formation.[32]
Kidney stones are more likely to develop, and to grow larger, if a person has low dietary magnesium. Magnesium inhibits stone formation.[33]
Animal protein
Diets in Western nations typically contain a large proportion of animal protein. Eating animal protein creates an acid load that increases urinary excretion of calcium and uric acid and reduced citrate. Urinary excretion of excess sulfurous amino acids (e.g., cysteine and methionine), uric acid, and other acidic metabolites from animal protein acidifies the urine, which promotes the formation of kidney stones.[34] Low urinary-citrate excretion is also commonly found in those with a high dietary intake of animal protein, whereas vegetarians tend to have higher levels of citrate excretion.[28] Low urinary citrate, too, promotes stone formation.[34]
Vitamins
The evidence linking vitamin C supplements with an increased rate of kidney stones is inconclusive.[35][36] The excess dietary intake of vitamin C might increase the risk of calcium-oxalate stone formation.[37] The link between vitamin D intake and kidney stones is also tenuous. Excessive vitamin D supplementation may increase the risk of stone formation by increasing the intestinal absorption of calcium; correction of a deficiency does not.[28]
Other
There are no conclusive data demonstrating a cause-and-effect relationship between alcoholic beverage consumption and kidney stones. However, some people have theorized that certain behaviors associated with frequent and binge drinking can lead to dehydration, which can, in turn, lead to the development of kidney stones.[38]
The American Urological Association has projected that global warming will lead to an increased incidence of kidney stones in the United States by expanding the "kidney stone belt" of the southern United States.[39]
In one study, people with lymphoproliferative/myeloproliferative disorders who were treated with chemotherapy developed symptomatic kidney stones 1.8% of the time.[40]
Prevention
Preventative measures depend on the type of stones. In those with calcium stones, drinking lots of fluids, thiazide diuretics and citrate are effective as is allopurinol in those with high uric acid levels in the blood or urine.[75][76]
Dietary measures
Specific therapy should be tailored to the type of stones involved. Diet can have an effect on the development of kidney stones. Preventive strategies include some combination of dietary modifications and medications with the goal of reducing the excretory load of calculogenic compounds on the kidneys.[29][77][78] Dietary recommendations to minimize the formation of kidney stones include:
Increasing total fluid intake to more than two liters per day of urine output.[79]
Limiting cola, including sugar-sweetened soft drinks,[75][79][80] to less than one liter per week.[81]
Limiting animal protein intake to no more than two meals daily (an association between animal protein and recurrence of kidney stones has been shown in men[82]).
Maintenance of dilute urine by means of vigorous fluid therapy is beneficial in all forms of kidney stones, so increasing urine volume is a key principle for the prevention of kidney stones. Fluid intake should be sufficient to maintain a urine output of at least 2 litres (68 US fl oz) per day.[76] A high fluid intake has been associated with a 40% reduction in recurrence risk.[52] The quality of the evidence for this, however, is not very good.[76]
Calcium binds with available oxalate in the gastrointestinal tract, thereby preventing its absorption into the bloodstream, and reducing oxalate absorption decreases kidney stone risk in susceptible people.[83] Because of this, some doctors recommend chewing calcium tablets during meals containing oxalate foods.[84] Calcium citrate supplements can be taken with meals if dietary calcium cannot be increased by other means. The preferred calcium supplement for people at risk of stone formation is calcium citrate because it helps to increase urinary citrate excretion.[78]
Aside from vigorous oral hydration and eating more dietary calcium, other prevention strategies include avoidance of large doses of supplemental vitamin C and restriction of oxalate-rich foods such as leaf vegetables, rhubarb, soy products and chocolate.[85] However, no randomized, controlled trial of oxalate restriction has been performed to test the hypothesis that oxalate restriction reduces stone formation.[84] Some evidence indicates magnesium intake decreases the risk of symptomatic kidney stones.[85]
Urine alkalinization
The mainstay for medical management of uric acid stones is alkalinization (increasing the pH) of the urine. Uric acid stones are among the few types amenable to dissolution therapy, referred to as chemolysis. Chemolysis is usually achieved through the use of oral medications, although in some cases, intravenous agents or even instillation of certain irrigating agents directly onto the stone can be performed, using antegrade nephrostomy or retrograde ureteral catheters.[43] Acetazolamide is a medication that alkalinizes the urine. In addition to acetazolamide or as an alternative, certain dietary supplements are available that produce a similar alkalinization of the urine. These include sodium bicarbonate, potassium citrate, magnesium citrate, and Bicitra (a combination of citric acid monohydrate and sodium citrate dihydrate).[86] Aside from alkalinization of the urine, these supplements have the added advantage of increasing the urinary citrate level, which helps to reduce the aggregation of calcium oxalate stones.[43]
Increasing the urine pH to around 6.5 provides optimal conditions for dissolution of uric acid stones. Increasing the urine pH to a value higher than 7.0 increases the risk of calcium phosphate stone formation. Testing the urine periodically with nitrazine paper can help to ensure the urine pH remains in this optimal range. Using this approach, stone dissolution rate can be expected to be around 10 mm (0.4 in) of stone radius per month.[43]
Diuretics
One of the recognized medical therapies for prevention of stones is the thiazide and thiazide-like diuretics, such as chlorthalidone or indapamide. These drugs inhibit the formation of calcium-containing stones by reducing urinary calcium excretion.[11] Sodium restriction is necessary for clinical effect of thiazides, as sodium excess promotes calcium excretion. Thiazides work best for renal leak hypercalciuria (high urine calcium levels), a condition in which high urinary calcium levels are caused by a primary kidney defect. Thiazides are useful for treating absorptive hypercalciuria, a condition in which high urinary calcium is a result of excess absorption from the gastrointestinal tract.[45]
Allopurinol
For people with hyperuricosuria and calcium stones, allopurinol is one of the few treatments that have been shown to reduce kidney stone recurrences. Allopurinol interferes with the production of uric acid in the liver. The drug is also used in people with gout or hyperuricemia (high serum uric acid levels).[87] Dosage is adjusted to maintain a reduced urinary excretion of uric acid. Serum uric acid level at or below 6 mg/100 ml) is often a therapeutic goal. Hyperuricemia is not necessary for the formation of uric acid stones; hyperuricosuria can occur in the presence of normal or even low serum uric acid. Some practitioners advocate adding allopurinol only in people in whom hyperuricosuria and hyperuricemia persist, despite the use of a urine-alkalinizing agent such as sodium bicarbonate or potassium citrate.[43]
Treatment
Stone size influences the rate of spontaneous stone passage. For example, up to 98% of small stones (less than 5 mm (0.2 in) in diameter) may pass spontaneously through urination within four weeks of the onset of symptoms,[7] but for larger stones (5 to 10 mm (0.2 to 0.4 in) in diameter), the rate of spontaneous passage decreases to less than 53%.[73] Initial stone location also influences the likelihood of spontaneous stone passage. Rates increase from 48% for stones located in the proximal ureter to 79% for stones located at the vesicoureteric junction, regardless of stone size.[73] Assuming no high-grade obstruction or associated infection is found in the urinary tract, and symptoms are relatively mild, various nonsurgical measures can be used to encourage the passage of a stone.[43] Repeat stone formers benefit from more intense management, including proper fluid intake and use of certain medications, as well as careful monitoring.[88]
Pain management
Management of pain often requires intravenous administration of NSAIDs or opioids.[11] NSAIDs appear somewhat better than opioids or paracetamol in those with normal kidney function.[89] Medications by mouth are often effective for less severe discomfort.[56] The use of antispasmodics does not have further benefit.[9]
Medical expulsive therapy
The use of medications to speed the spontaneous passage of stones in the ureter is referred to as medical expulsive therapy.[90][91] Several agents, including alpha adrenergic blockers (such as tamsulosin) and calcium channel blockers (such as nifedipine), may be effective.[90] Alpha-blockers likely result in more people passing their stones, and they may pass their stones in a shorter time.[91] Alpha-blockers appear to be more effective for larger stones (over 5 mm in size) than smaller stones.[91] A combination of tamsulosin and a corticosteroid may be better than tamsulosin alone.[90] These treatments also appear to be a useful in addition to lithotripsy.[7]
Lithotripsy
Extracorporeal shock wave lithotripsy (ESWL) is a noninvasive technique for the removal of kidney stones. Most ESWL is carried out when the stone is present near the renal pelvis. ESWL involves the use of a lithotriptor machine to deliver externally applied, focused, high-intensity pulses of ultrasonic energy to cause fragmentation of a stone over a period of around 30–60 minutes. Following its introduction in the United States in February 1984, ESWL was rapidly and widely accepted as a treatment alternative for renal and ureteral stones.[92] It is currently used in the treatment of uncomplicated stones located in the kidney and upper ureter, provided the aggregate stone burden (stone size and number) is less than 20 mm (0.8 in) and the anatomy of the involved kidney is normal.[93][94]
For a stone greater than 10 mm (0.4 in), ESWL may not help break the stone in one treatment; instead, two or three treatments may be needed. Some 80 to 85% of simple renal calculi can be effectively treated with ESWL.[7] A number of factors can influence its efficacy, including chemical composition of the stone, presence of anomalous renal anatomy and the specific location of the stone within the kidney, presence of hydronephrosis, body mass index, and distance of the stone from the surface of the skin.[92] Common adverse effects of ESWL include acute trauma, such as bruising at the site of shock administration, and damage to blood vessels of the kidney.[95][96] In fact, the vast majority of people who are treated with a typical dose of shock waves using currently accepted treatment settings are likely to experience some degree of acute kidney injury.[92]
ESWL-induced acute kidney injury is dose-dependent (increases with the total number of shock waves administered and with the power setting of the lithotriptor) and can be severe,[92] including internal bleeding and subcapsular hematomas. On rare occasions, such cases may require blood transfusion and even lead to acute kidney failure. Hematoma rates may be related to the type of lithotriptor used; hematoma rates of less than 1% and up to 13% have been reported for different lithotriptor machines.[96] Recent studies show reduced acute tissue injury when the treatment protocol includes a brief pause following the initiation of treatment, and both improved stone breakage and a reduction in injury when ESWL is carried out at slow shock wave rate.[92]
In addition to the aforementioned potential for acute kidney injury, animal studies suggest these acute injuries may progress to scar formation, resulting in loss of functional renal volume.[95][96] Recent prospective studies also indicate elderly people are at increased risk of developing new-onset hypertension following ESWL. In addition, a retrospective case-control study published by researchers from the Mayo Clinic in 2006 has found an increased risk of developing diabetes mellitus and hypertension in people who had undergone ESWL, compared with age and gender-matched people who had undergone nonsurgical treatment. Whether or not acute trauma progresses to long-term effects probably depends on multiple factors that include the shock wave dose (i.e., the number of shock waves delivered, rate of delivery, power setting, acoustic characteristics of the particular lithotriptor, and frequency of retreatment), as well as certain intrinsic predisposing pathophysiologic risk factors.[92]
To address these concerns, the American Urological Association established the Shock Wave Lithotripsy Task Force to provide an expert opinion on the safety and risk-benefit ratio of ESWL. The task force published a white paper outlining their conclusions in 2009. They concluded the risk-benefit ratio remains favorable for many people.[92] The advantages of ESWL include its noninvasive nature, the fact that it is technically easy to treat most upper urinary tract calculi, and that, at least acutely, it is a well-tolerated, low-morbidity treatment for the vast majority of people. However, they recommended slowing the shock wave firing rate from 120 pulses per minute to 60 pulses per minute to reduce the risk of renal injury and increase the degree of stone fragmentation.[92]
Surgery
Most stones under 5 mm (0.2 in) pass spontaneously.[29][7] Prompt surgery may, nonetheless, be required in persons with only one working kidney, bilateral obstructing stones, a urinary tract infection and thus, it is presumed, an infected kidney, or intractable pain.[97] Beginning in the mid-1980s, less invasive treatments such as extracorporeal shock wave lithotripsy, ureteroscopy, and percutaneous nephrolithotomy began to replace open surgery as the modalities of choice for the surgical management of urolithiasis.[7] More recently, flexible ureteroscopy has been adapted to facilitate retrograde nephrostomy creation for percutaneous nephrolithotomy. This approach is still under investigation, though early results are favorable.[98] Percutaneous nephrolithotomy or, rarely, anatrophic nephrolithotomy, is the treatment of choice for large or complicated stones (such as calyceal staghorn calculi) or stones that cannot be extracted using less invasive procedures.[47][7]
Ureteroscopic surgery
Ureteroscopy has become increasingly popular as flexible and rigid fiberoptic ureteroscopes have become smaller. One ureteroscopic technique involves the placement of a ureteral stent (a small tube extending from the bladder, up the ureter and into the kidney) to provide immediate relief of an obstructed kidney. Stent placement can be useful for saving a kidney at risk for postrenal acute kidney failure due to the increased hydrostatic pressure, swelling and infection (pyelonephritis and pyonephrosis) caused by an obstructing stone. Ureteral stents vary in length from 24 to 30 cm (9.4 to 11.8 in) and most have a shape commonly referred to as a "double-J" or "double pigtail", because of the curl at both ends. They are designed to allow urine to flow past an obstruction in the ureter. They may be retained in the ureter for days to weeks as infections resolve and as stones are dissolved or fragmented by ESWL or by some other treatment. The stents dilate the ureters, which can facilitate instrumentation, and they also provide a clear landmark to aid in the visualization of the ureters and any associated stones on radiographic examinations. The presence of indwelling ureteral stents may cause minimal to moderate discomfort, frequency or urgency incontinence, and infection, which in general resolves on removal. Most ureteral stents can be removed cystoscopically during an office visit under topical anesthesia after resolution of urolithiasis.[99]
More definitive ureteroscopic techniques for stone extraction (rather than simply bypassing the obstruction) include basket extraction and ultrasound ureterolithotripsy. Laser lithotripsy is another technique, which involves the use of a holmium:yttrium aluminium garnet (Ho:YAG) laser to fragment stones in the bladder, ureters, and kidneys.[100]
Ureteroscopic techniques are generally more effective than ESWL for treating stones located in the lower ureter, with success rates of 93–100% using Ho:YAG laser lithotripsy.[73] Although ESWL has been traditionally preferred by many practitioners for treating stones located in the upper ureter, more recent experience suggests ureteroscopic techniques offer distinct advantages in the treatment of upper ureteral stones. Specifically, the overall success rate is higher, fewer repeat interventions and postoperative visits are needed, and treatment costs are lower after ureteroscopic treatment when compared with ESWL. These advantages are especially apparent with stones greater than 10 mm (0.4 in) in diameter. However, because ureteroscopy of the upper ureter is much more challenging than ESWL, many urologists still prefer to use ESWL as a first-line treatment for stones of less than 10 mm, and ureteroscopy for those greater than 10 mm in diameter.[73] Ureteroscopy is the preferred treatment in pregnant and morbidly obese people, as well as those with bleeding disorders.[7]
Medically reviewed by
Dr. Rabeya Afroz Shomi
MBBS, FCPS, Dhaka Medical
3 Years of Experience
- Written by the Priyojon Editorial Team
